Padeken Lab
Research Publications Group Members BiographyRegulation of heterochromatin in response to genotoxic stress
The epigenetic memory of a cell is shaped by pathways that establish, erase and maintain chromatin marks. Lysine 9 methylation on histone H3 (H3K9me) is a defining modification of heterochromatin. In multicellular eukaryotes, heterochromatin has two main functions. First, H3K9me silences the transcription of satellite repeats and transposable elements to ensure genome stability. Secondly, it silences tissue-specific genes during development and maintains stable differentiated states. The unprogrammed transcription of repetitive DNA elements leads to an accumulation of toxic R-loops and genomic instability. Thus, it is not surprising that loss of appropriately targeted heterochromatin is associated with cancer, loss of tissue integrity and ageing.
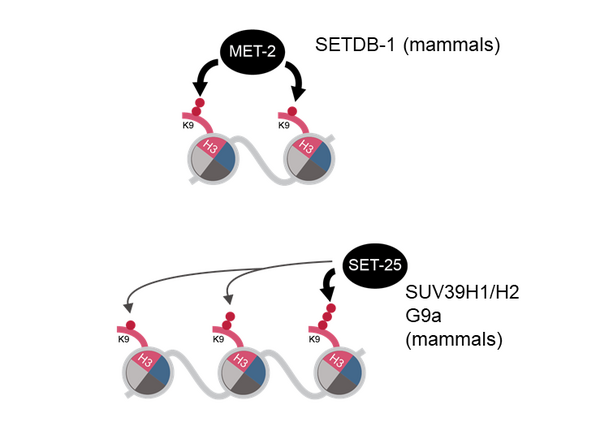
Multiple pathways mediate the establishment and targeting of H3K9me and heterochromatin (Figure 1). However, despite its crucial importance in the maintenance of genomic and tissue integrity, we do not yet understand what controls heterochromatin establishment in complex organisms. These pathways are of particular interest, because on the one hand it has been shown in yeast that H3K9me is sufficient to induce its own propagation through the cell cycle, which under stress conditions can result in the semi-stable establishment of “epi-mutations” (ectopic, heritable gene silencing). On the other hand, mistargeted H3K9me has been observed in several cancer models, potentially contributing to metastasis formation and cancer progression through the silencing of tumour suppressor genes. In addition, recent studies identify H3K9-specific histone methyltransferases (HMTs) as mediators of immune escape in anti-PD-1/CTLA-4 treated tumours.
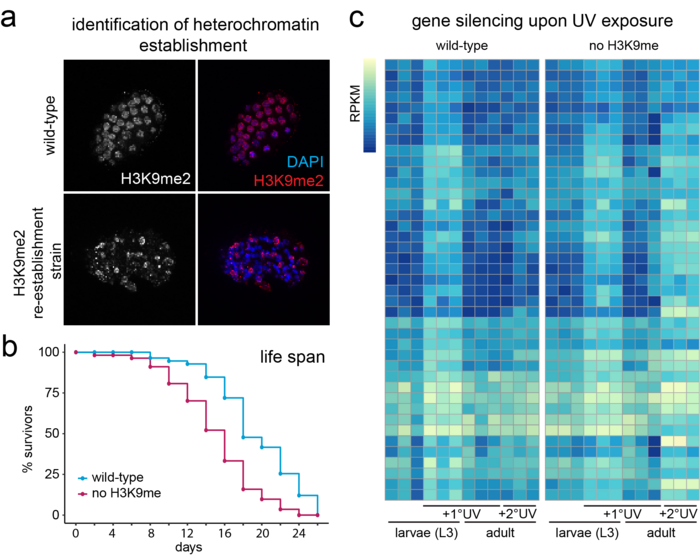
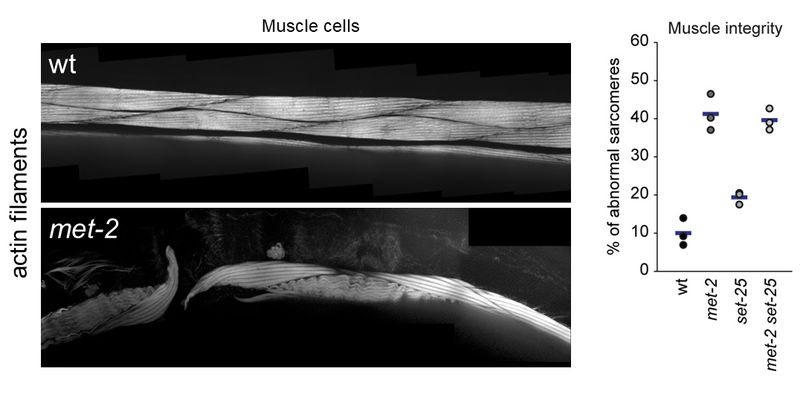
Our research explores how heterochromatin is altered upon exposure to persistent genotoxic stress and how this contributes to the adaptation of cells/tumours to their environment, as well as genomic and tissue integrity (Figures 2 and 3). We do this using C. elegans as a disease model in combination with powerful genetic tools, next-generation sequencing and quantitative microscopy to characterise acute and long-term changes in heterochromatin. This will allow us to discover how the epigenetic response to DNA damage impacts human diseases such as cancer, ageing and Cockayne syndrome.
Recruiting
We are looking for a highly motivated team players with a biological background and an interest in genome-wide epigenetic profiling techniques, CRISPR and quantitative microscopy. If you would like to join our team, please contact us by email.